Sierra Biopharma is Developing Antigen-Specific Therapy Targeting Autoimmune Diseases
Sierra Biopharma, a biotechnology startup founded on intellectual property developed by researchers at the University of California, Davis, is developing a new therapeutic approach to treat autoimmune diseases.
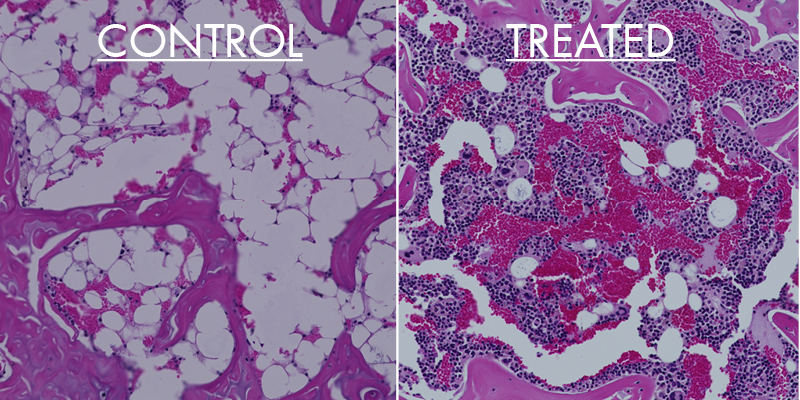
Inventors and co-founders Robert Fairclough and Vu Trinh have set an initial focus on treating myasthenia gravis, a chronic autoimmune disease that affects 1.4 million people globally. Myasthenia gravis causes use-induced muscle fatigue and generalized muscle weakness that can result in life-altering difficulties with seeing, swallowing, talking and walking. The disease stems from a mistake made by the immune system in producing antibodies that bind to neurotransmitter receptors, triggering the immune system to destroy the folded post-synaptic muscle fiber membranes that are vital for repeated muscle contractions.
Sierra Biopharma is taking a new approach to treat the disease with an antigen-specific therapy that attacks the cause of the disease without suppressing the entire immune system. To accomplish this, the company is developing a biologic compound that not only binds to, neutralizes and clears the pathogenic antibodies, but also binds to and eliminates the memory B-cells responsible for producing more of the pathogenic antibodies.
Fairclough and Trinh recently completed the Biotech Innovation Gallery (BIG) Accelerator program led by UC Davis Venture Catalyst. The program provides leaders of UC Davis-associated biotech startups with training on how to develop an effective business model, strategies to protect intellectual property and guidance on how to pitch to potential strategic partners and investors.. The year’s program concluded with a showcase event where 22 startups, including Sierra Biopharma, pitched their value proposition to venture capitalists and biotech companies attending the annual J.P. Morgan Healthcare Conference in San Francisco.
“It was amazing to be surrounded by so many fantastic biotech startups from UC Davis,” said Fairclough, an emeritus associate professor in the UC Davis Department of Neurology. “We were able to pitch our research to so many VCs and companies and they provided us with great insight and guidance in how best to proceed with the company’s development.”
Sierra Biopharma is now scaling up production of the therapeutic biologic and plans to complete the proof of concept and preclinical work in the next six months. The company has accepted an invitation to participate in the 2020 Science2Startup showcase event — a forum for top scientists from around the world to present their ideas and interact with leading investors and executives in the Boston biotechnology hub.